
Unlocking the therapeutic potential of fibroblasts
Discoveries made by our founders and other leading researchers in recent years show that fibroblasts, a cell type long known for their structural role in both health and disease, have a far-reaching influence and control on the primary players of the immune system, including T cells, B cells, dendritic cells and macrophages. Fibroblasts are important players in organizing and regulating immune responses in the local inflammatory environments.
Mestag utilizes its unique understanding of fibroblast biology to drug important fibroblast-immune biology, including TLS induction and stromally-driven inhibitor receptors.
In addition, we use our Reversing Activated Fibroblast Technology (RAFT) platform, a proprietary platform purposely built to model the role of pathogenic fibroblasts in human disease, to identify novel drug targets. Our approach is underpinned by a deep understanding of modeling fibroblast biology in a disease-relevant context, highly specialist data analytics, and complex and tailored human tissue multi-cellular co-culture assays.
Developing new therapies for patients with cancer and inflammatory disease
![]() Tertiary Lymphoid Structures (TLSs) to treat cancer:
Tertiary Lymphoid Structures (TLSs) to treat cancer:
The presence of TLSs in tumors has recently been shown to be strongly predictive of both significantly improved patient outcomes and better responses to therapy for cancer patients across multiple solid tumor types. These striking observations have been seen in several thousand cancer patients, indicating the remarkable therapeutic potential of inducing TLSs1,2,3,4.
Activated fibroblasts play important roles in shaping the tumor microenvironment (TME) and depending on context, may inhibit or stimulate potent anti-tumor immune responses in cancer. When fibroblastic reticular cells (FRCs), specialized fibroblasts found in the TME, are activated through the body’s natural immune response, they produce chemokines that drive the creation of high endothelial venules (HEV) and TLSs.
M300 is a bispecific antibody designed to conditionally induce TLSs in tumors by agonizing lymphotoxin beta receptor (LTBR) in the tumor on co-engagement of fibroblast activation protein (FAP). LTBR is a member of the TNF-receptor super family and is the essential driver of lymphoid structure development. Our FAP-LTBR bispecific antibody, demonstrates robust anti-tumor responses in preclinical models as monotherapy and in combination with checkpoint inhibitors, ADC and radiotherapy.
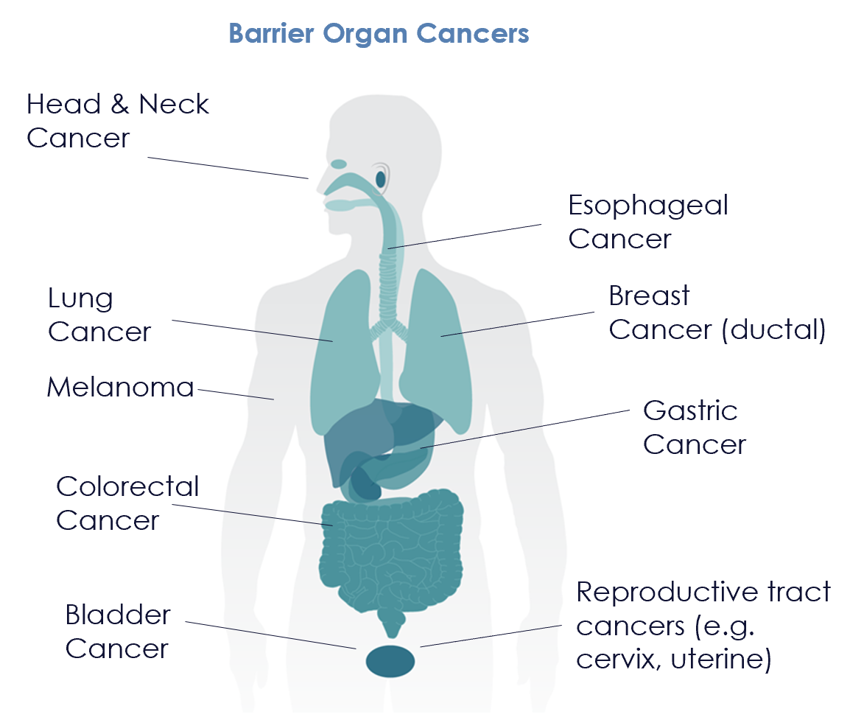
The M300 program is rapidly advancing towards its first clinical trial in cancer patients, with a focus on tumors in barrier organs. Collectively, these tumors account for 340.000 cancer deaths each year in the US5.
![]()
Agonist antibodies to treat autoimmune and inflammatory disease: Inflammation is a vital defensive reaction by the immune system to injury and pathogens. However, chronic, unresolved inflammation is associated with multiple severe diseases. There are over 65 million cases in the world of the top six major immune-mediated chronic inflammatory diseases — asthma, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, psoriasis, and atopic dermatitis6.
In chronic inflammatory diseases, activated fibroblasts influence immune activity both directly and indirectly, in addition to playing a key role in structural remodeling and tissue integrity.
Our M402 stromal agonist program is designed to dampen the pro-inflammatory processes of the immune system, to achieve durable remission for inflammatory disease patients. We are particularly excited by the role myeloid biology plays in perpetuating chronic autoimmune and inflammatory states, and have leveraged state-of-the-art antibody design to ensure optimal agonism within the active synapse.
Tertiary Lymphoid Structures (TLSs)
Advancing therapies to patients
TLSs are aggregates of immune cells that form as part of our bodies’ natural immune response to infection or cancer, and drive powerful immune responses by recruiting, educating, and activating new immune cells. TLSs are a normal defense mechanism against foreign antigen, forming dynamically locally in infected tissues, acting as “immune system SWAT teams” to ensure infections are cleared rapidly.
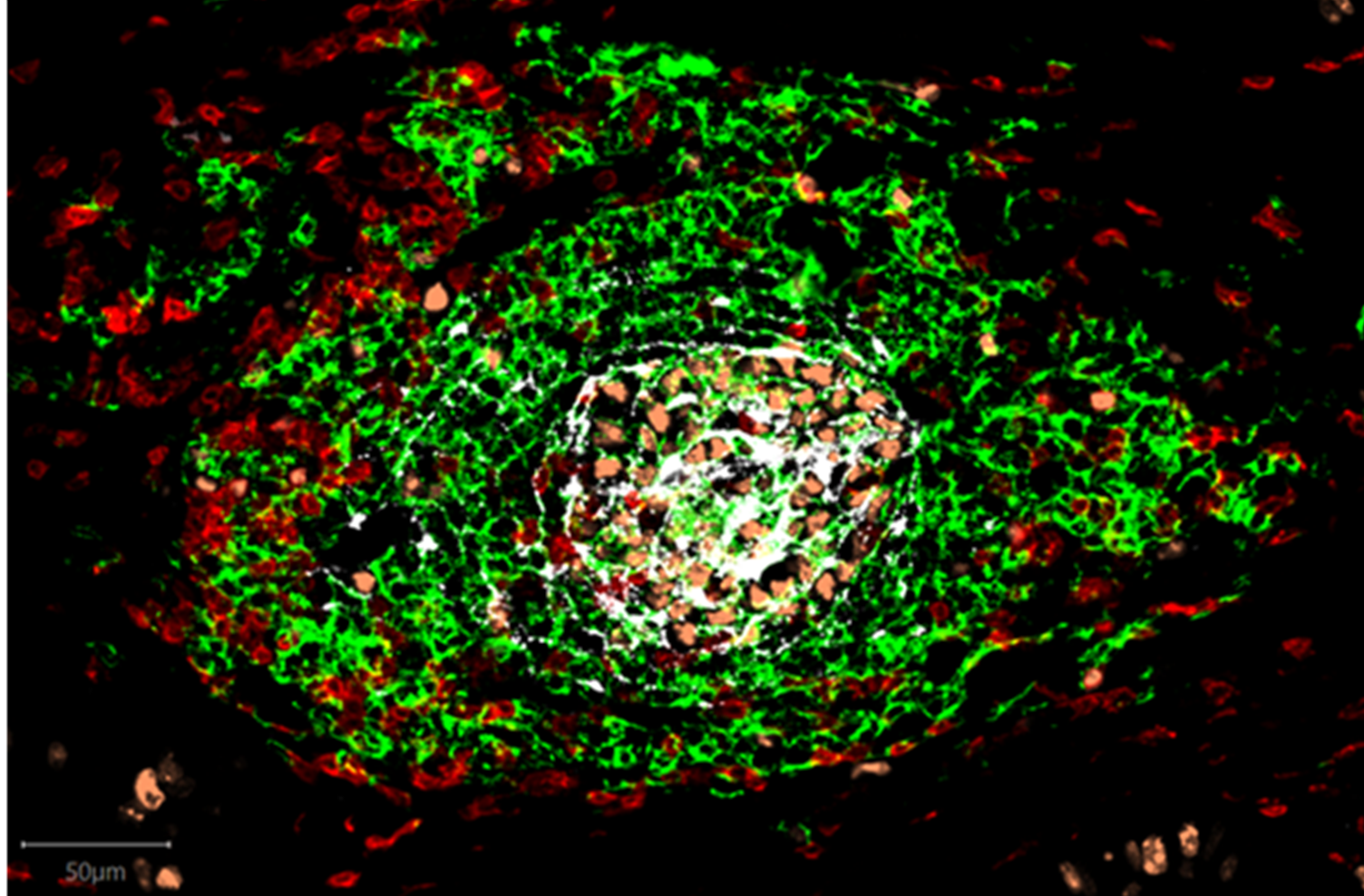
Tertiary Lymphoid Structure (TLS)
The presence of TLSs in tumors has recently been shown to be strongly predictive of both significantly improved patient outcomes and better responses to therapy for cancer patients across multiple solid tumor types. These striking observations have been seen in several thousand cancer patients, indicating the remarkable therapeutic potential of inducing TLSs1,2,3,4.
TLSs are associated with benefit to patients irrespective of their tumor’s PDL1 expression status7 and have also been shown to extend the duration of response to checkpoint inhibitors8.
TLSs form during chronic inflammatory conditions in response to foreign antigen and form part of the body’s local response to cancer or infection. TLSs are organized lymphoid aggregates consisting of T-cells, B-cells, Dendritic Cells and Fibroblast Reticular Cells (specialized fibroblasts responsible for organizing TLSs) and share many functional and structural features with secondary lymphoid organs, in particular lymph nodes. TLSs drive antigen-specific immune responses and are equipped with specialized blood vessels known as high endothelial venules (HEVs) that act as lymphocyte highways and facilitate the migration of lymphocytes from the blood into tissue9. Like TLSs, HEVs correlate strongly with cancer patient survival and response to therapies10.
TLSs are believed to drive potent anti-tumor immune responses via enhanced immune cell access to the tumor through the formation of high endothelial venules and robust immune cell education via local antigen presentation and immune cell priming, leading to a powerful orchestrated anti-tumor response11. Both B-cell and T-cell responses are enhanced through TLS formation12.
TLSs can be visualized in tumors, where they form within and close to tumor tissue, driving local anti-tumor responses.